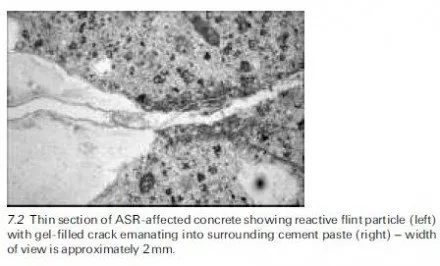
Briefly, alkali-silica reaction is a reaction between the alkali hydroxides in the pore solution of concrete and thermodynamically unstable silica in certain aggregates. The reaction product is an alkali-silica gel (of varying composition) which has the tendency to imbibe water and swell. The swelling pressures can be sufficient to induce expansion and cracking of the concrete. Figure 7.2 shows a photomicrograph of a thin section prepared from ASR-affected concrete. The bottom left-hand corner of the image shows a sand-size particle of reactive flint. The particle has reacted with the alkalis, expanded and cracked, and the crack extends out from the aggregate into the surrounding hydrated cement matrix to the right of the particle. The crack is partially filled with alkali-silica gel. Figure 7.3 shows how this reaction manifests itself in an unreinforced concrete wall. The internal expansion results in randomly distributed cracks at the exposed surface; this phenomenon is often termed pattern cracking or map cracking.,
 Despite its name, the alkali-silica reaction is actually a reaction between the hydroxyl ions (OHÿ) in the pore solution and certain siliceous components of the aggregate; the reactive silica is not directly attacked by the alkali metal cations (Na+ and K+). When poorly crystalline hydrous silica is exposed to a strong alkaline solution, there is an acid-base reaction between the hydroxyl ions in solution and the acidic silanol (Si-OH) groups (Dent Glasser and Kataoka, 1981) as follows:
Despite its name, the alkali-silica reaction is actually a reaction between the hydroxyl ions (OHÿ) in the pore solution and certain siliceous components of the aggregate; the reactive silica is not directly attacked by the alkali metal cations (Na+ and K+). When poorly crystalline hydrous silica is exposed to a strong alkaline solution, there is an acid-base reaction between the hydroxyl ions in solution and the acidic silanol (Si-OH) groups (Dent Glasser and Kataoka, 1981) as follows:

reserves of alkali hydroxide are available, the process continues to produce an alkaline silicate solution.
Despite general acceptance of the chemical reactions involved, a number of different mechanisms of expansion have been proposed. Hansen’s (1944) osmotic theory regards the cement paste surrounding reactive grains as a semi- permeable membrane through which water (or pore solution) may pass but not the larger complex silicate ions. The water is drawn into the reacting grain where its chemical potential is lowest. An osmotic pressure cell is formed and increasing hydrostatic pressure is exerted on the cement paste, inevitably leading to cracking of the surrounding mortar.
Hansen’s laboratory experiments confirmed that osmotic pressures were generated by sodium silicate solutions when separated from water by a cement paste membrane. Indeed, this is the principle behind the osmotic cell test for assessing aggregate reactivity (Stark, 1983) In this test, adjacent cells, filled with sodium hydroxide solution, are separated by a cement paste membrane; a sample of aggregate is introduced into one cell and the rate of osmotic flow (into the cell containing the aggregate) provides a measure of aggregate reactivity.
McGowan and Vivian (1952) disputed the classical osmotic theory on the basis that cracking of the surrounding cement paste `membrane’ due to ASR would relieve hydraulic pressure and prevent further expansion. Their work showed that expansion was accompanied by the formation and widening of cracks due to mechanical (absorption) rather than hydraulic forces. They proposed an alternative mechanism based on the physical absorption of water by the alkali silica-gel and subsequent swelling of the gel. Tang (1981) concurred with the water imbibition and swelling theory; his observations of polished sections suggested that expansion occurs before the reaction product becomes fluid. Thus, the presence and composition of a semi-permeable membrane is insignificant.
Powers and Steinour (1955a, 1955b) proposed a compromise, suggesting that both osmotic and imbibition pressures may be generated depending on whether the alkali-silicate complex is fluid or solid. In their hypothesis, the reaction product itself may act as a semi-permeable membrane depending on its composition. Regardless of the mechanism, the fundamental cause of swelling is thermodynamically the same, i.e. the entry of water into a region where the effect of a solute or of adsorption reduces its free energy.