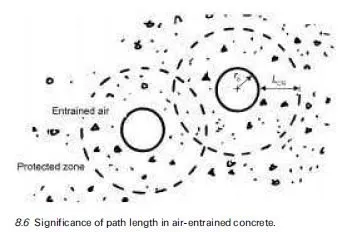
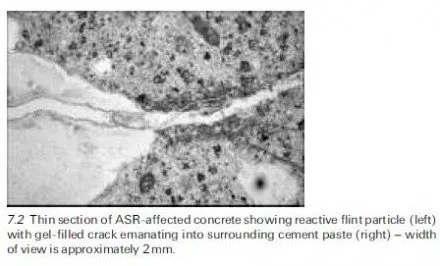
The ability of lithium-based compounds to suppress deleterious expansion due to alkali-silica reaction (ASR) in mortar and concrete was first demonstrated by McCoy and Caldwell (1951) over 50 years ago. They showed that, out of more than 100 chemical compounds tested, various salts of lithium (e.g. LiCl, Li2CO3, LiF, Li2SiO3, LiNO3, and Li2SO4) were the most promising and could virtually eliminate the expansion of mortar containing Pyrex glass provided they were used in sufficient quantity. Since then, there have been numerous studies, most con- ducted in the last decade or so, which corroborate this earlier discovery. Although most lithium compounds have a beneficial effect, recent work has indicated that lithium nitrate (LiNO3) is the most efficient form for suppressing ASR as its incorporation in concrete does not result in a significant augmentation of the pore solution hydroxyl concentration (Stokes et al., 1997). Lithium nitrate solution is now marketed by a number of companies in North America as an `ASR-sup- pressing admixture’. In all cases, the product is sold as a 30% solution of LiNO3.
It is somewhat paradoxical that lithium compounds are effective suppressants of ASR as lithium is an alkali metal like sodium and potassium. The precise mechanism by which lithium controls ASR is not known, although many theories have been put forward (Feng et al., 2005). The simplest and most commonly used explanation is that lithium salts will interact with reactive silica in a similar way to sodium and potassium salts, but the reaction product is an insoluble lithium-silicate with little propensity to imbibe water and swell.
The initial work of McCoy and Caldwell (1951) showed that the amount of lithium required to control expansion was a function of the availability of other alkalis (Na + K) in the system and they concluded that the expansion of mortar bars containing reactive Pyrex glass could be effectively suppressed provided that the lithium-to-sodium-plus-potassium molar ratio was greater that 0.74, i.e. [Li]/[Na+K] > 0.74. Since then numerous workers have demonstrated a similar relationship between the amount of lithium required and the amount of alkali available, but the minimum value of [Li]/[Na+K] has been shown to vary depending on a number of issues such as the form of lithium, nature of reactive aggregate and, perhaps, the method of test used (Feng et al., 2005).
Recent research (Tremblay et al., 2004) has highlighted the influence of aggregate type on the amount of lithium required to suppress expansion due to ASR. In this study, which included 12 reactive aggregates from sources in Canada, the [Li]/[Na+K] ratio required to prevent the expansion of concrete prisms exceeding 0.04% after 2 years storage at 38ëC was between 0.56 to 0.74 for 6 of the 12 aggregates and 0.93 to 1.11 for 3 aggregates. The ratio of [Li]/ [Na+K] =ˆ 1.11 was not sufficient for the remaining 3 aggregates.
Currently, concrete expansion tests are considered to be the most reliable laboratory test methods for evaluating lithium-based compounds and deter- mining the amount of lithium required with a specific aggregate. Although, a number of modifications have been proposed to the (ultra) accelerated mortar bar test to render it suitable for use with lithium compounds, there are few data available to assess the reliability of the modified tests.
Guidelines on the use of lithium compounds for preventing ASR in new construction or retarding expansion in existing ASR-affected structures have been published by the Federal Highways Administration in the USA (Folliard et al., 2003).