Plain carbon steels can be given a great range of properties by heat treatment and by working; but addition of alloying elements greatly extends those properties or makes the heat-treating operations easier and simpler. For example, combined high tensile strength and toughness, corrosion resistance, high-speed cutting, and many other specialized purposes require alloy steels. However, the most important effect of alloying is the influence on hardenability.
Effects of Alloying Elements
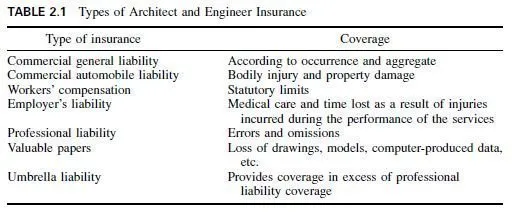
Important alloying elements from the standpoint of building, and their principal effects, are summarized below:
Aluminum restricts grain growth during heat treatment and promotes surface hardening by nitriding.
Chromium is a hardener, promotes corrosion resistance (see Art. 4.44.2), and promotes wear resistance.
Copper promotes resistance to atmospheric corrosion and is sometimes combined with molybdenum for this purpose in low-carbon steels and irons. It strengthens steel and increases the yield point without unduly changing elongation or reduction of area.
Manganese in low concentrations promotes hardenability and nondeforming, nonshrinking characteristics for tool steels. In high concentrations, the steel is austenitic under ordinary conditions, is extremely tough, and work-hardens readily. It is therefore used for teeth of power-shovel dippers, railroad frogs, rock crushers, and similar applications.
Molybdenum is usually associated with other elements, especially chromium and nickel. It increases corrosion resistance, raises tensile strength and elastic limit without reducing ductility, promotes casehardening, and improves impact resistance.
Nickel boosts tensile strength and yield point without reducing ductility; increases low-temperature toughness, whereas ordinary carbon steels become brittle;
promotes casehardening; and in high concentrations improves corrosion resistance under severe conditions. It is often used with chromium (see Art. 4.44.2). Invar contains 36% nickel.
Silicon strengthens low-alloy steels; improves oxidation resistance; with low carbon yields transformer steel, because of low hysteresis loss and high permeability;
in high concentrations provides hard, brittle castings, resistant to corrosive chemicals, useful in plumbing lines for chemical laboratories.
Sulfur promotes free machining, especially in mild steels.
Titanium prevents intergranular corrosion of stainless steels by preventing grainboundary depletion of chromium during such operations as welding and heat treatment.
Tungsten, vanadium, and cobalt are all used in high-speed tool steels, because they promote hardness and abrasion resistance. Tungsten and cobalt also increase high-temperature hardness.
The principal effects of alloying elements are summarized in Table 4.19.
Stainless Steels
Stainless steels of primary interest in building are the wrought stainless steels of the austenitic type. The austenitic stainless steels contain both chromium and nickel.
Total content of alloy metals is not less than 23%, with chromium not less than 16% and nickel not less than 7%. Commonly used stainless steels have a tensile strength of 75 ksi and yield point of 30 ksi when annealed. Cold-finished steels may have a tensile strength as high as 125 ksi with a yield point of 100 ksi.
Austenitic stainless steels are tough, strong, and shock-resistant, but work-harden readily; so some difficulty on this score may be experienced with cold working and machining. These steels can be welded readily but may have to be stabilized (e.g., AISI Types 321 and 347) against carbide precipitation and intergranular corrosion due to welding unless special precautions are taken. These steels have the best high-temperature strength and resistance to scaling of all the stainless steels.
Types 303 and 304 are the familiar 18-8 stainless steels widely used for building applications. These and Types 302 and 316 are the most commonly employed stainless steels. Where maximum resistance to corrosion is required, such as resistance to pitting by seawater and chemicals, the molybdenum-containing Types 316 and 317 are best.
For resistance to ordinary atmospheric corrosion, some of the martensitic and ferritic stainless steels, containing 15 to 20% chromium and no nickel, are employed.
The martensitic steels, in general, range from about 12 to 18% chromium and from 0.08 to 1.10% carbon. Their response to heat treatment is similar to that of the plain carbon steels. When chromium content ranges from 15 to 30% and carbon content is below 0.35%, the steels are ferritic and nonhardenable. The highchromium steels are resistant to oxidizing corrosion and are useful in chemical plants.