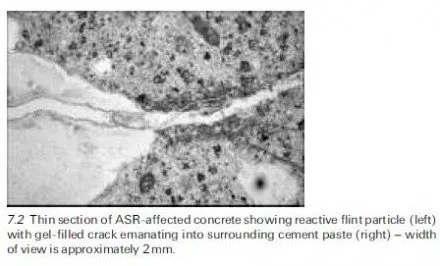
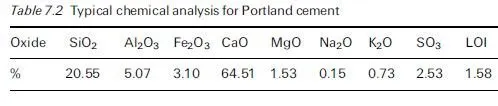
The capillary pore water contains solutes, in particular alkalis and salts. The ice crystals that grow as the temperature drops below freezing point are formed from pure water. This drives the solutes into surrounding unfrozen pore water. This water contains both existing solutes and those driven from the now frozen pure water. There is an increase in solute level leading to a concentration gradient.
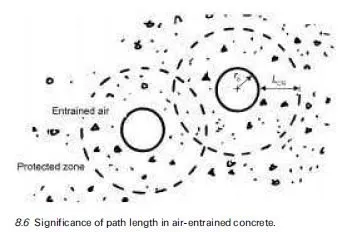
Further growth of ice crystals in a capillary pore has a dual effect. Firstly, as the volume of ice increases, it expels water from the capillary to the gel pore network. Secondly, it leads to a continual increase in the concentration gradient. The resultant osmotic pressure eventually becomes a significant driving force and unfrozen water, including gel pore water, diffuses towards the developing ice body. The phenomenon is outlined in Fig. 8.5. Thus even concrete that is not fully saturated is at risk. The self-feeding growth of the ice body as temperatures fall may result in dilation of the pore cavity and development of potentially deleterious expansive pressures (Helmuth, 1960).