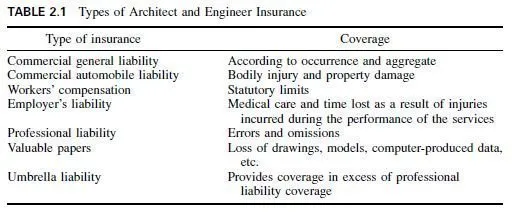
Pure aluminum and aluminum alloys are used in buildings in various forms. Highpurity aluminum (at least 99% pure) is soft and ductile but weak. It has excellent corrosion resistance and is used in buildings for such applications as bright foil for heat insulation, roofing, flashing, gutters and downspouts, exterior and interior architectural trim, and as pigment in aluminum-based paints. Its high heat conductivity recommends it for cooking utensils. The electrical conductivity of the electrical grade is 61% of that of pure copper on an equal-volume basis and 201% on an equal-weight basis.
Aluminum alloys are generally harder and stronger than the pure metal. Furthermore, pure aluminum is difficult to cast satisfactorily, whereas many of the alloys are readily cast.
Pure aluminum is generally more corrosion resistant than its alloys. Furthermore, its various forms pure and alloy have different solution potentials; that is, they are anodic or cathodic to each other, depending on their relative solution potentials.
A number of alloys are therefore made with centers or cores of aluminum alloys, overlaid with layers of metal, either pure aluminum or alloys, which are anodic to the core. If galvanic corrosion conditions are encountered, the cladding metal protects the core sacrifically.