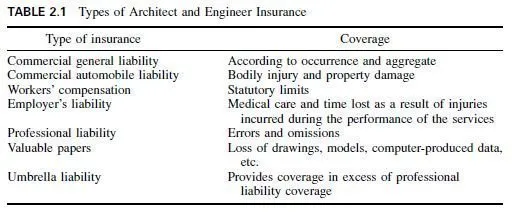
These are prepared by fusing a mixture of aluminous and calcareous materials (usually bauxite and limestone) and grinding the resultant product to a fine powder.
These cements are characterized by their rapid-hardening properties and the high strength developed at early ages. Table 4.3 shows the relative strengths of 4-in cubes of 1:2:4 concrete made with normal portland, high-early-strength portland, and aluminous cements.
Since a large amount of heat is liberated with rapidly by aluminous cement during hydration, care must be taken not to use the cement in places where this heat cannot be dissipated. It is usually not desirable to place aluminous-cement concretes in lifts of over 12 in; otherwise the temperature rise may cause serious weakening of the concrete.
Aluminous cements are much more resistant to the action of sulfate waters than are portland cements. They also appear to be much more resistant to attack by water containing aggressive carbon dioxide or weak mineral acids than the silicate cements. Their principal use is in concretes where advantage may be taken of their very high early strength or of their sulfate resistance, and where the extra cost of the cement is not an important factor.
Another use of aluminous cements is in combination with firebrick to make refractory concrete. As temperatures are increased, dehydration of the hydration products occurs. Ultimately, these compounds create a ceramic bond with the aggregates.