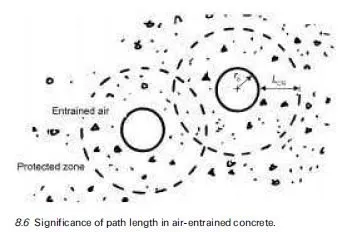
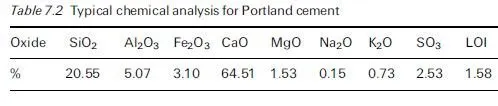
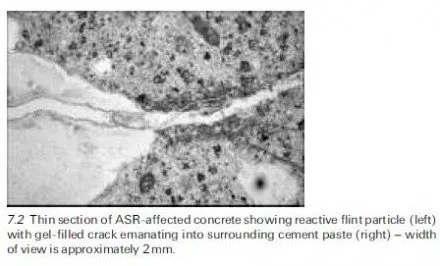
In general, the rates of attack by aggressive chemicals on concrete may be affected by several factors, including the following: · The concentrations of the reacting chemicals and their rates of replenishment in the aqueous medium surrounding the concrete. · In the case of acids, the concentration of H+ ions, determined by their strengths, i.e. their dissociation constants (Ka), and by the overall concen- tration of the acid concerned. (Note: mineral acids, such as H2SO4, are highly ionised and so, at the same overall concentration, attack concrete at a greater rate than do the partially ionised weak acids, such as carbonic acid.) · The solubility products (Ksp) of the reaction products and their abilities to form insoluble protective films.