Masonry mortar holds masonry units together, and also holds them apart (compensates for their dimensional tolerances). Mortar for unit masonry is addressed by ASTM C270, which in turn cites other ASTM specifications.
Specifying masonry mortar under ASTM C270 requires three choices:
The designer must choose a cementitious system. Three options are possible: cement-lime mortar, masonry-cement mortar, or mortar-cement mortar.
The designer must choose a mortar type, basically related to the proportion of cement in the mortar.
The designer must choose whether ASTM C270 will be enforced by proportions of the different ingredients, or by the properties of the final mortar. The proportion specification is the default, and is assumed to govern if the designer does not state otherwise.
Each choice is discussed in more detail in Sec. 2.2.5. For now, to help explain the background and significance of these choices, it is useful to discuss the chemistry of masonry mortar.
Introduction to the Chemistry of Masonry Mortar
Masonry mortars can be broadly classified as sand-lime mortars and hydraulic mortars. The former harden (set) only in the presence of air.
The latter can harden under water.
Chemistry of Sand-Lime Mortar
Since the time of the Romans, masonry mortar has been made from a mixture of lime and sand. Limestone is first calcined (heated) to produce quicklime (calcium oxide). The chemical formula for this reaction, and its corresponding verbal explanation, is shown below:
Limestone + heat = calcium oxide + carbon dioxide (quicklime)
CaCO3 = CaO + CO2
To form mortar, the quicklime is mixed with water to produce hydrated lime, plus large amounts of heat:
Calcium oxide + water = calcium hydroxide + heat
(quicklime) (hydrated lime)
CaO + H2O = Ca(OH)2 + heat
Finally, exposure to the at mosphere converts the calcium hydroxide to calcium carbonate. This reaction takes place over several years:
Calcium hydroxide + air = calcium carbonate + water
(hydrated lime) (carbon dioxide) (limestone)
Ca(OH)2 + CO2 = CaCO3 + H2O
Sand-lime mortar is found in many historic buildings. It hardens very slowly, but also has the ability to deform slowly over time without cracking. Sand-lime mortar is not a hydraulic-cement mortar because the last step in its hardening process (conversion of calcium hydroxide to calcium carbonate) occurs only in the presence of air.
Chemistry of Hydraulic-Cement Mortars Hydraulic cements harden as a result of a chemical reaction of minerals with water. Hydraulic cements have been used since prehistoric times. Their cementitious ingredients include pozzolanic cements, gypsum cements, portland cements, and other cements.
Chemistry of Pozzolanic CementsThese were discovered by the Greeks. The word pozzolan comes from a site in Italy (Pozzuoli, near the volcano Vesuvius) where these minerals were found and used by the Romans. A pozzolan possesses little or no cementitious properties on its own, but reacts with calcium hydroxide and water to form cementitious compounds. An example of a natural pozzolan is quartz, whose chemical formula (SiO2 â‹… XH2O) denotes silica (silicon dioxide) combined chemically with water. When finely ground quartz is mixed with hydrated lime [calcium hydroxide, or Ca(OH)2], the following reaction occurs:
SiO2 â‹… XH2O + Ca(OH)2 = Ca1-3SiO3 â‹… H2O
(calcium silicate, a natural cement)
Chemistry of Gypsum Cement Plaster of Paris Gypsum reacts much faster with water than lime or pozzolans do. Pure gypsum sets in about 5 min.
Commercial gypsum (such as Hydrostone®) sets in about 45 min because it has a retarder with it.
Gypsum rock is calcined (heated) like limestone, but requires less energy:
CaSO4 â‹… 2H2O + heat = CaSOH4 â‹… 1/2H2O + 3/2H2O
(gypsum rock) (plaster of Paris)
When water is added to the calcined gypsum, it reverts to its original state:
CaSO4 â‹… 1/2H2O + 3/2H2O = CaSOH4 â‹… 2H2O + heat
(plaster of paris) (gypsum rock)
The resulting cement is very strong and stiff as concrete. Its main disadvantage is that it expands slowly over time as it absorbs water from the outside air. This produces large splitting forces if the gypsum is restrained.
In the calcining operation, if the gypsum rock is heated too much, the following undesirable reaction results, producing a powder that is not useful for building:
CaSO4 â‹… 2H2O + heat = CaSOH4 + 2H2O
(gypsum rock) (anhydrite)
Chemistry of Portland Cement
Portland cement is a particular class of hydraulic cement. It was first manufactured in England in the early 1800s, and was so named because its color was thought to resemble that of a natural limestone from the Isle of Portland.
Hardened portland cement is the result of the hydration of four principal chemical constituents:
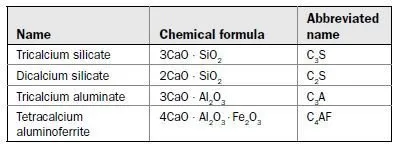
Dry (unhydrated) cement consists of these compounds in powdered form. When water is added, the compounds combine with water in an exothermic (heat-producing) reaction, to form calcium hydroxide (about 25% by weight) and calcium silicate hydrate (about 50% by weight).
Chemistry of Other Hydraulic Cements In recent years, portland cement has increasingly been used in combination with other hydraulic cements, particularly pozzolanic cements and slag cements. Each has its own ASTM specification. Pozzolanic cements combine with calcium hydroxide to produce calcium silicate hydrate. Slag cements (usually produced from ground granulated blast-furnace slag or GGBF slag) are combinations of silicates and aluminosilicates. Slag cements, when hydrated, produce primarily calcium silicate hydrates as well.
Cementitious Systems Used in Modern Masonry Mortar
Modern masonry mortar is composed of cementitious agents (portland cement or other hydraulic cements and hydrated lime, or masonry cement, or mortar cement), sand, and water. Each of these can be referred to as a cementitious system. Three cementitious systems are defined by ASTM C270:
Cement and lime
Masonry cement
Mortar cement
The first of these (cement and lime) is self-explanatory. The second and third (masonry cement and mortar cement) are generally mixtures of portland or blended cement and plasticizing materials (such as hydrated lime or finely ground limestone), together with other materials introduced to enhance performance. These other materials generally include air-entraining and water-retention additives, intended to improve freezethaw durability, workability, and water retention. These are discussed further in this chapter.