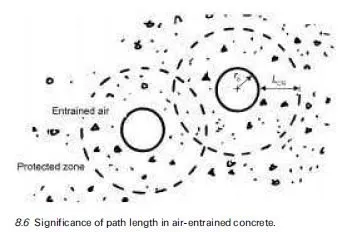
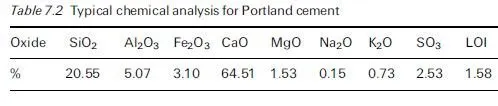
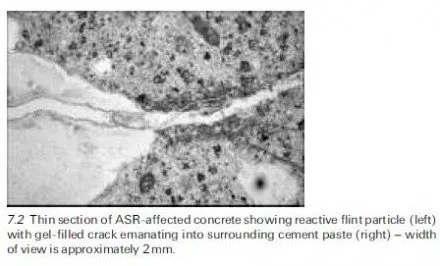
Silica, SiO2, is a component of many rocks; however, not all forms of silica react significantly with the pore solution of concrete. For example, quartz is a very stable silica mineral owing to the fact that it has a well-ordered crystalline structure. Opal, on the other hand, has a more disordered (amorphous) structure, despite having the same chemical composition as quartz (i.e. SiO2). Alkali-silica reaction does not occur in concrete produced with pure quartz sand; indeed, quartz sand is used as the standard aggregate in mortar tests conducted on cement. ASR will occur rapidly in concrete produced with an aggregate con- taining opaline silica, provided there is sufficient alkali present, and this reaction can, under certain circumstances result in expansion and cracking of the concrete.
The following silica minerals have been shown to react deleteriously in concrete: opal, tridymite, cristobalite, volcanic glass, chert, cryptocrystalline (or microcrystalline) quartz and strained quartz. These minerals may be found in the following rock types: shale, sandstone, silicified carbonate rocks, chert, flint, quartzite, quartz-arenite, gneiss, argillite, granite, greywacke, siltstone, arenite, arkose and hornfels. However, this does not mean that all sources of such rocks will produce deleterious reaction when used in concrete. For example, granitic aggregate is widely used in concrete and only certain sources produce damaging ASR. The reactivity of a rock depends on the type and quantity of reactive minerals present, if any. The presence of reactive minerals can usually be detected by a trained petrographer. However, appropriate performance testing of specific aggregate sources is recommended to confirm alkali-silica reactivity. Test methods are discussed in Section 7.6.