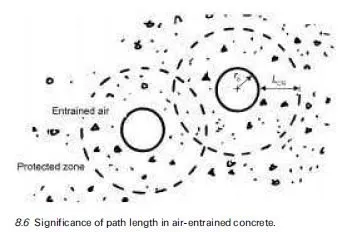
Severe scaling of concrete surfaces has been associated in many instances with the application of deicing agents. These agents are spread on snow and ice to form a solution with a lower freezing point than water so that melting occurs earlier. Deicing agents may be chloride based. These deicing salts are notorious for their deleterious effect on the passivity of embedded reinforcement (see Chapter 5) but equally they may cause surface scaling. This is because deicing agents can increase the degree of saturation of the concrete they are in contact with, may encourage differential response of layers to freezing, may subject concrete surface layers to thermal shock and may exacerbate osmotic pressure. These factors are discussed further in this section.
The degree of saturation of concrete is dependent on the rate of water take-up and the rate of evaporation. The degree of saturation at a given temperature and external relative humidity is higher in salt-contaminated pore water than in concrete free from the influence of deicing salts. Thus for typical drying conditions a greater proportion of the pore structure retains water and has a high degree of saturation.
The differential response of layers to freezing temperatures in concrete regularly exposed to deicing agents is due to the ingress of melt water from previous cycles. Even in well drained pavements and decks, the melt water which contains deicing agent will penetrate the surface and make its way into the layers just below the surface. The impregnated layers will have a depressed freezing point in comparison with water-soaked layers. Over time rainwater washes deicing agent from the uppermost surface layers, which may then contain purer water than those below at time of next freeze. Differential behaviour of these surface layers may occur leading to the expansive outer layer breaking free with consequent scaling.
Thermal shock is an inevitable consequence of the action of deicing agent in fulfilling its role. The melting of ice or snow on a pavement surface through the application of salt requires energy. This energy is drawn from the concrete and this leads to a rapid temperature drop in the layers of concrete near the surface.
The situation is temporary but the consequent thermal shock may cause stresses in the concrete thus leading to cracking and scaling. The distribution of deicing agent in concrete is not even because its entry is via the external surface. This uneven distribution becomes more pronounced during freezing cycles because the ice formation will be accompanied by rejection of deicing chemicals leading to higher concentration differences. This increases osmotic pressure. The contribution of osmotic pressure to ice crystal growth, referred to in Section 8.2, will clearly be exacerbated if deicing salt application increases the solute concentration gradient in the pore water (Neville, 1995).