Although a large amount of data have been obtained for a range of polymer- cement composite properties, relatively little is known, apart from that discussed in Section 10.2.4, about the nature of polymer-cement interfaces and interactions that may take place between the two components. In the field of composites generally, it is widely accepted that interfaces between different components are important in determining properties such as strength and durability. It is reasonable to assume that this will also be true in the case of polymer-cement composites. Studying the interface between the cement and polymer phases in a com- posite involves a number of practical difficulties. As the cement phase is porous, it is quite probable that polymer will penetrate surface pores resulting in a complex and diffuse interfacial layer. The actual interfacial region itself is hard to reveal and if interaction products have been formed, amounts may be very small and difficult to extract and analyse separately from the cement or polymer phases. Different specimen geometries may be mechanically loaded with the aim of achieving at least some interfacial failure thus permitting subsequent surface examination of the interfacial region. This is, however, difficult to achieve in practice even with carefully notched samples with the locus of failure often passing through bulk material. An alternative method is the selective dissolution of either the polymer or cement phase, in theory leaving the interfacial region available for surface analysis. In addition, the amounts of interaction products may be magnified by increasing the surface area, e.g. using a powdered cement rather than trying to cast against a flat surface. A number of systems have been investigated (Short, 1999; Shaw, 1989) with these points in mind, concentrating on the case when the monomer, methyl methacrylate (MMA), comes into contact with hydrated cements and is subsequently cured to give poly(methyl methacrylate) (PMMA). It was found that the monomeric MMA reacted with the cement to form primarily calcium methacrylate. This reaction proceeds in two stages:

Since the calcium methacrylate formed is highly water soluble, a reaction such as this would be generally detrimental to the properties of a polymer± cement composite. Water is a limiting reagent in these reactions and the amount of methacrylate formed is proportional to the initial amount of water present in the cement. In practice, if the highly water-soluble methacrylate were leached from the polymer±cement interface over time then the properties of the composite would be modified. This confirms the need for thorough drying of the cement or aggregate prior to contact with the monomer if adhesion is to be maximised.
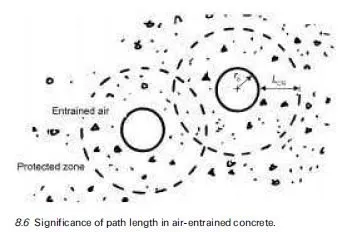
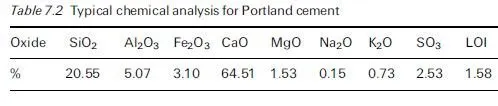
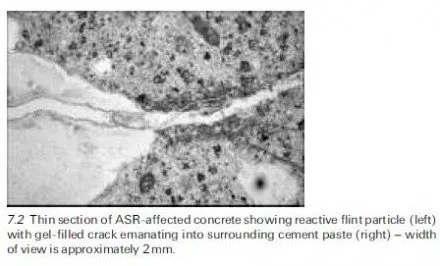
This is very useful……………,