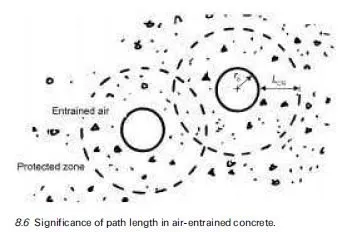
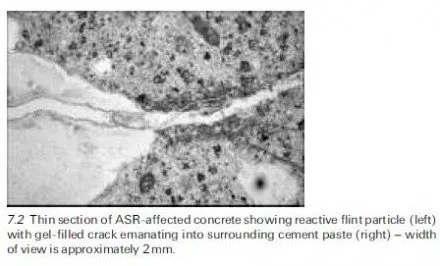
It follows from the above considerations that there is no unique threshold proportion of chloride contamination that applies generally and, in view of the wide disparity between the behaviour often recorded for laboratory-made and site-produced concrete, chloride limits for use in specification of concrete mix materials have had to be based on engineering judgement, taking account of knowledge of the condition of existing structures. This retrospective approach of course suffers from the drawback that it cannot be applied directly to deal with new construction incorporating innovative materials because the relevant long- term service records are simply not available. There is therefore pressure to devise rapid methods of determining chloride thresholds applicable to concrete made with different cements, admixtures, etc. Research in this area has not yet produced a general consensus, however, and the cautionary tale of calcium chloride, which was widely used over several decades as a cheap and effective accelerating admixture, should serve as a warning to those of a cavalier disposition with regard to chloride limits.
In the UK until the mid-1970s, the former British Standards Institution Code of Practice for the Structural Use of Concrete (CP110:1972) permitted calcium chloride to be used as an admixture in reinforced concrete at levels of up to 1.5% CaCl2 by weight of cement (equivalent to 0.96% Clÿ by weight of cement). Similar limits on the levels of chloride allowed in concreting materials were also imposed at that time in several other countries (Ramachandran, 1976). Owing to the widespread problems of chloride-induced corrosion encountered in practice, an amendment to CP110 was introduced in May 1977 requiring much tighter controls on the total levels of chloride arising from admixtures, aggregates or other internal sources. This effectively stopped the deliberate introduction of calcium chloride admixtures to concrete containing steel reinforcement, prestressing steel or other embedded metal.
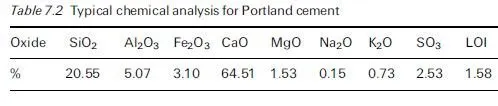
The new European Standard BS EN 206-1 (2000) has maintained the proscription on deliberate use of chloride-based admixtures and introduced various classes of maximum chloride content from all sources (corresponding to 0.1, 0.2 and 0.4% Clÿ by mass of cement) applicable to concrete containing steel reinforcement or other embedded metal (apart from corrosion-resistant lifting devices). They are used in accordance with the provisions of complementary national standards such as BS 8500-1:2002, that are intended to apply to situations of different perceived risk, the most obvious case for special concern being prestressed concrete, for which a maximum of 0.1% chloride is allowed. Similarly in BS 8500-1:2002, a limit of 0.2% chloride is specified for reinforced concrete made from Sulfate Resisting Portland Cement, which is required to contain no more than 3.5% C3A. The C3A component of Portland cement has long been known to play a significant role in binding chloride ions in the form of sparingly soluble chloro-aluminate hydrates (Roberts, 1962; Richartz, 1969) so reducing the proportion remaining in the pore solution phase. The level of C3A is therefore believed to be one of the influences accounting for the ability of different Portland cements to sustain different rates of corrosion at constant modest levels of chloride addition (Page et al., 1986; Masslehuddin et al., 1996). There are, however, several factors besides C3A content that affect chloride binding by different cements under different conditions (Larsen, 1998; Wowra and Setzer, 2000) and we do not yet have a clear understanding of the ways in which these influence the supply of free chloride ions to incipient anodic sites on steel in concrete.
In summing up this section, it is fair to say that, while many attempts have been made to quantify the various risk factors that determine the wide scatter of corrosion initiation probabilities represented at a given total chloride level (BRE, 2000b), there has been limited progress in this area. This is clearly illustrated in a recent review of chloride thresholds (Glass and Buenfeld, 1997) and reflects the fact that the threshold chloride content is not dominated by any one parameter, which could provide a simple `index’ for comparing different types of concrete. It is actually a function of interacting variables that include, but are not limited to, factors that affect the potential of the steel and those that influence the composition and microstructure of the interfacial zone of the concrete in the immediate vicinity of the embedded metal. This implies that there is often no straightforward answer to questions as to whether, for instance, particular formulations of blended or composite cement, used in conjunction with specific admixtures, will produce chloride threshold values significantly different from those found for other more traditional types of reinforced con- crete. Exposure tests of reasonably long duration, performed under conditions that are as realistic as possible, are required to decide these issues and electrochemical corrosion monitoring techniques of the sorts to be considered in Section 5.8 can be helpful if used appropriately in conjunction with such tests.