The fact that appreciable corrosion of steel does not usually happen in dense concrete is primarily due to the alkalinity of the cement which, as discussed in Chapter 2, typically results in the pore liquid phase of hardened cement having a pH value in the range 13±14 owing to the presence of dissolved NaOH and KOH. When steel is exposed to alkaline aqueous solutions at pH > 11.5 in the presence of dissolved oxygen (or even at more mildly alkaline pH values if the solutions concerned are buffered), the metal forms a surface oxide layer, some nanometres thick, termed a passive film. This layer serves as a barrier to the anodic iron dissolution process and reduces the corrosion rate of the steel to an imperceptible level, corresponding to an average rate of reduction in metal thickness of less than 1 um per year.
The effectiveness of the passive film in limiting the rate of anodic dissolution of steel may be illustrated by comparing experimentally observed relationships between applied potential and current density for steel anodes in typical neutral and alkaline solutions. These relationships, known as anodic polarisation curves (Page, 1988), indicate that, whereas in most aqueous solutions of pH 7 or lower, the rate of the anodic reaction:
Fe –> Fe^^2+ + 2^e-
increases exponentially as the potential of the steel anode is raised over a range of several hundred millivolts, this so-called `active dissolution’ phenomenon is not observed in aqueous solutions of pH > 11.5. Instead, the onset of formation of a passive film is marked by a sharp fall in the anodic current density when the potential is raised above a critical value (ca. ÿ700 mV, SCE scale) at which a film-forming reaction is initiated:
![]()
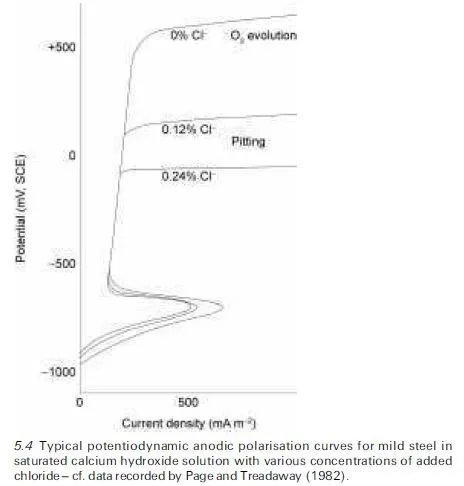
Thereafter very little change in the anodic current density with further increase in the steel potential is observed as the passive film merely thickens very slowly until another critical potential is reached at which oxygen gas starts to be produced by electrolytic decomposition of water, behaviour that is illustrated in Fig. 5.4 for steel in saturated calcium hydroxide solution without chloride addi- tion (pH ~ 12.5). This characteristic form of anodic polarisation curve, which signifies that passivity of the metal can be induced, has been shown experimentally to apply both to steel anodes in alkaline solutions and to those in hardened cement pastes, see, for example, Page and Treadaway (1982).