The following practical tests are made on limestones to determine their suitability:
(i) Physical tests
(ii) Heat test
(iii) Chemical test
(iv) Ball test.
(i) Physical Test: Pure limestone is white in colour. Hydraulic limestones are bluish grey, brown or are having dark colours. The hydraulic lime gives out earthy smell. They are having clayey taste. The presence of lumps give indication of quick lime and unburnt lime stones.
(ii) Heat Test: A piece of dry stone weighing W1 is heated in an open fire for few hours. If weight
of sample after cooling is W2, the loss of weight is W2 W1. The loss of weight indicates the amount of carbon dioxide. From this the amount of calcium carbonate in limestone can be worked out.
(iii) Chemical Test: A teaspoon full of lime is placed in a test tube and dilute hydrochloric acid is poured in it. The content is stirred and the test tube is kept in the stand for 24 hours. Vigourous effervescence and less residue indicates pure limestone. If effervescence is less and residue is more it indicates impure limestone.
If thick gel is formed and after test tube is held upside down it is possible to identify class of lime as indicated below:
Class A lime, if gel do not flow.
Class B lime, if gel tends to flow down.
Class C lime, if there is no gel formation.(iv) Ball Test: This test is conducted to identify whether the lime belongs to class C or to class B.
By adding sufficient water about 40 mm size lime balls are made and they are left undisturbed for six hours. Then the balls are placed in a basin of water. If within minutes slow expansion and slow disintegration starts it indicates class C lime. If there is little or no expansion, but only cracks appear it belongs to class B lime.
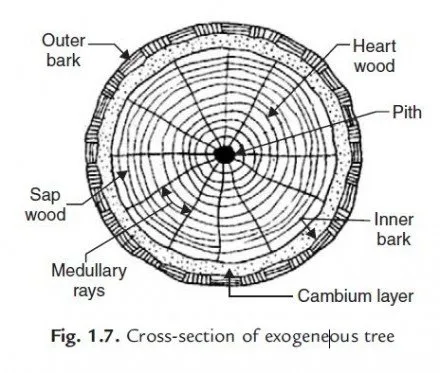
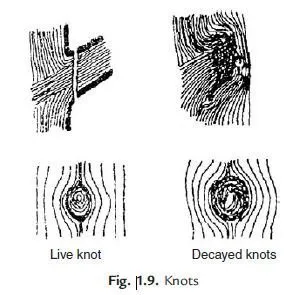
Many thanks for the series of tests and their results given in the article. These can provide a valuable reference for civil engineers, researchers, and anyone interested in understanding the properties and behavior of limestone in construction.